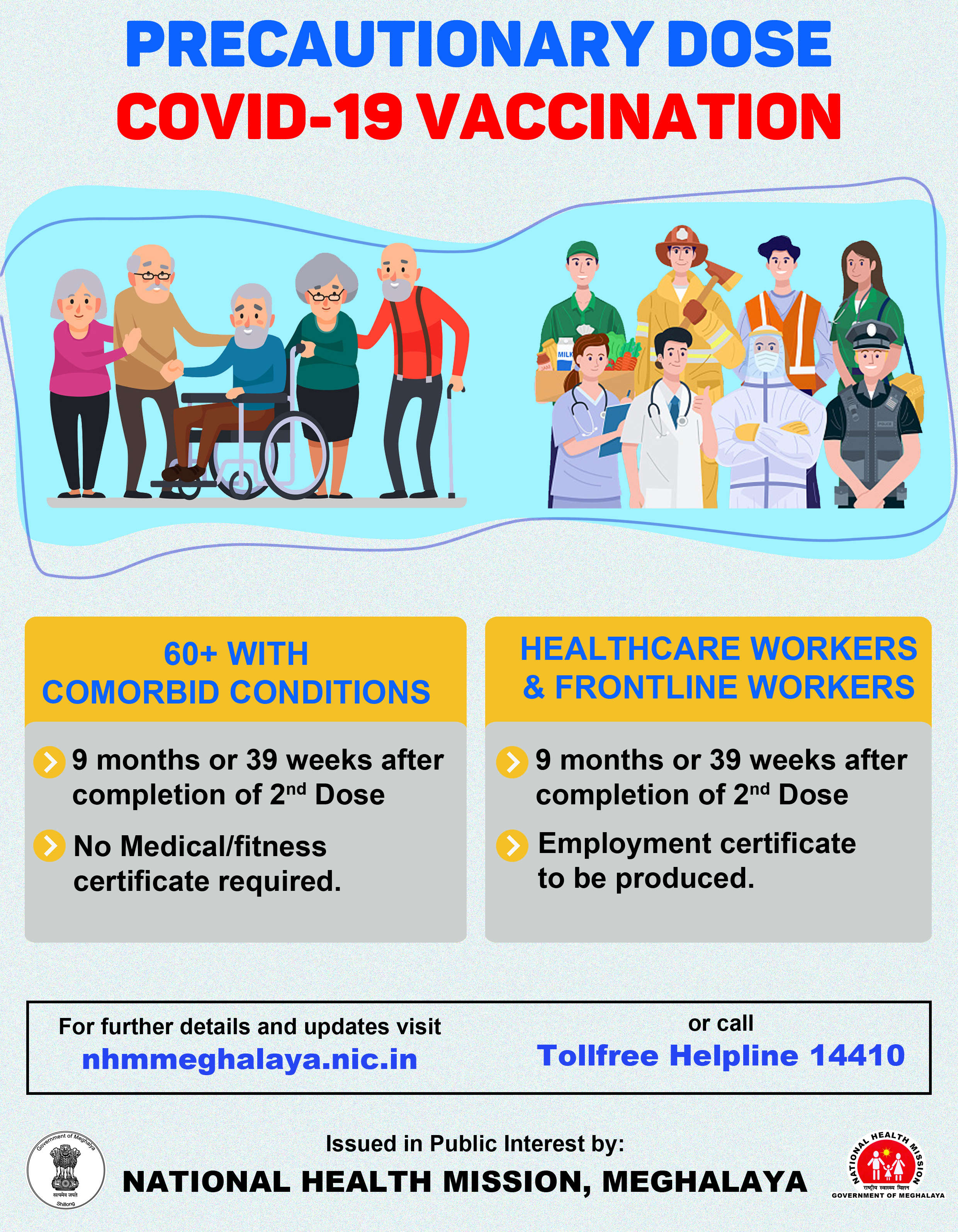
Bharat Biotech begins trials for third booster dose of Covaxin at AIIMS
The clinical trials of the third dose of indigenous COVID-19 vaccine, Covaxin, as a booster dose, six months after administering the second dose, has begun today at All India Institute of Medical Science (AIIMS), Delhi.
"The booster shot will be given to individuals who had enrolled for the Phase II trials. This is being done not only at AIIMs but at various centres across the country to see if a third booster shot improves the immunity after the two doses of vaccine," Dr Puneet Misra, professor of community medicine at AIIMS, said.
"The idea is to compare the immunity and other parameters of those who have taken both shots and others who will be given a booster dose," Misra added.
The study will be conducted on 190 participants who were already vaccinated with COVAXIN (as part of the phase 2 trial) six months ago. There will be nine sites where the study for booster dose will be conducted on participants between the age group of 18 to 55 years.
Earlier, Bharat Biotech had got permission from the expert panel of the Drug Controller General of India to conduct clinical trials for the third dose of Covaxin.
(Edited by Ridhika Joshi)
TNT-The Northeast Today is now on WhatsApp. CLICK HERE to receive more updates on your phone.