UK approves Oxford-AstraZeneca Covid-19 vaccine for emergency use
LONDON
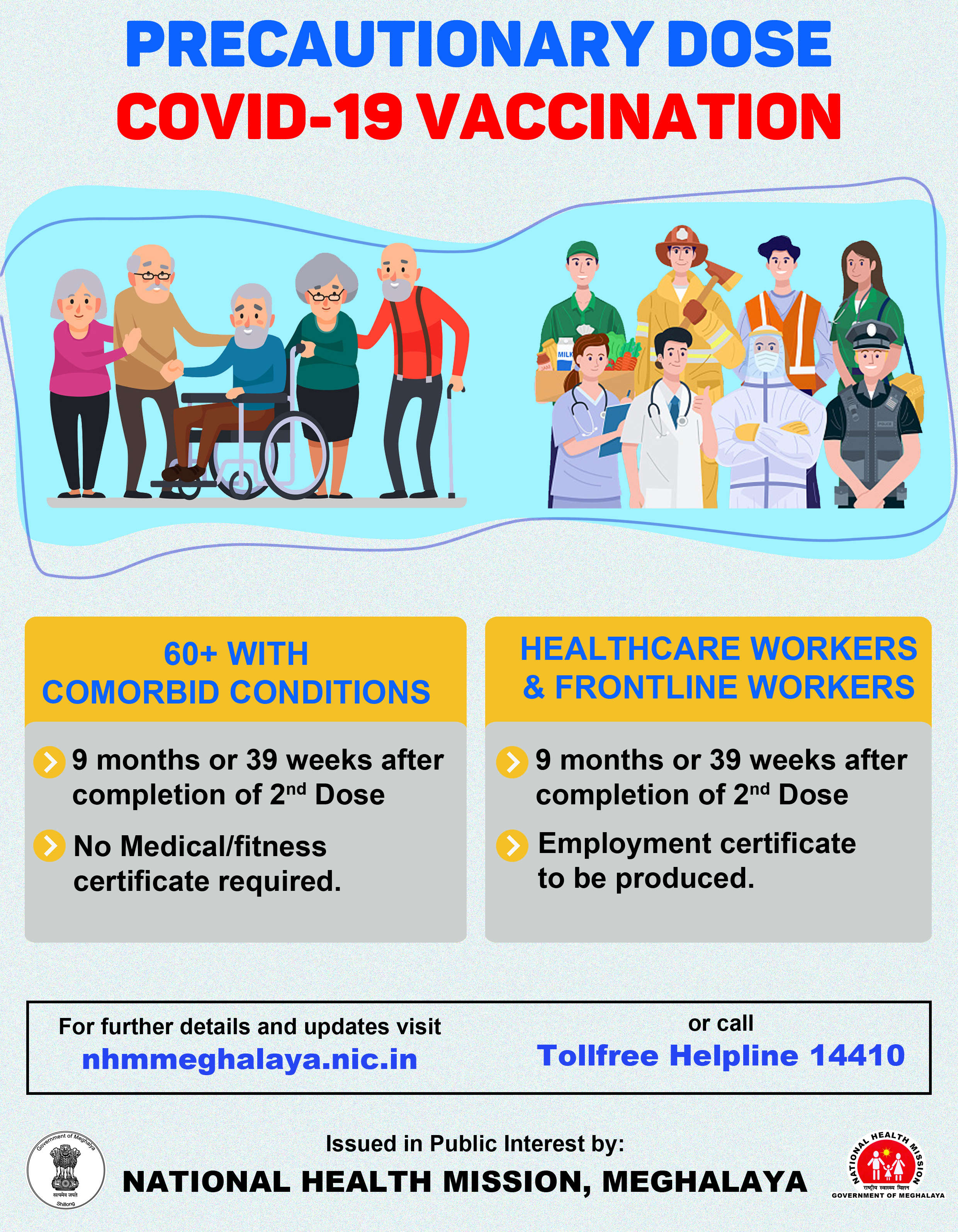
The United Kingdom on December 30 approved the Oxford-AstraZeneca Covid-19 vaccine for emergency use amidst rising cases of the new contagious variant of the virus. .
The UK government accepted the recommendation from the Medicines and Health Care Products Regulatory Agency (MHRA), and the rollout is expected to begin as early as next week.
AstraZeneca, in a statement said, “The Company aims to supply millions of doses in the first quarter as part of an agreement with the government to supply up to 100 million doses in total”.
With this, the UK has become the first country to approve the vaccination for mass usage starting early next year.
The MHRA decision to approve the vaccine was based on successive trails of the vaccine over the last few months.
(Edited by Shankar Kumar Turha)