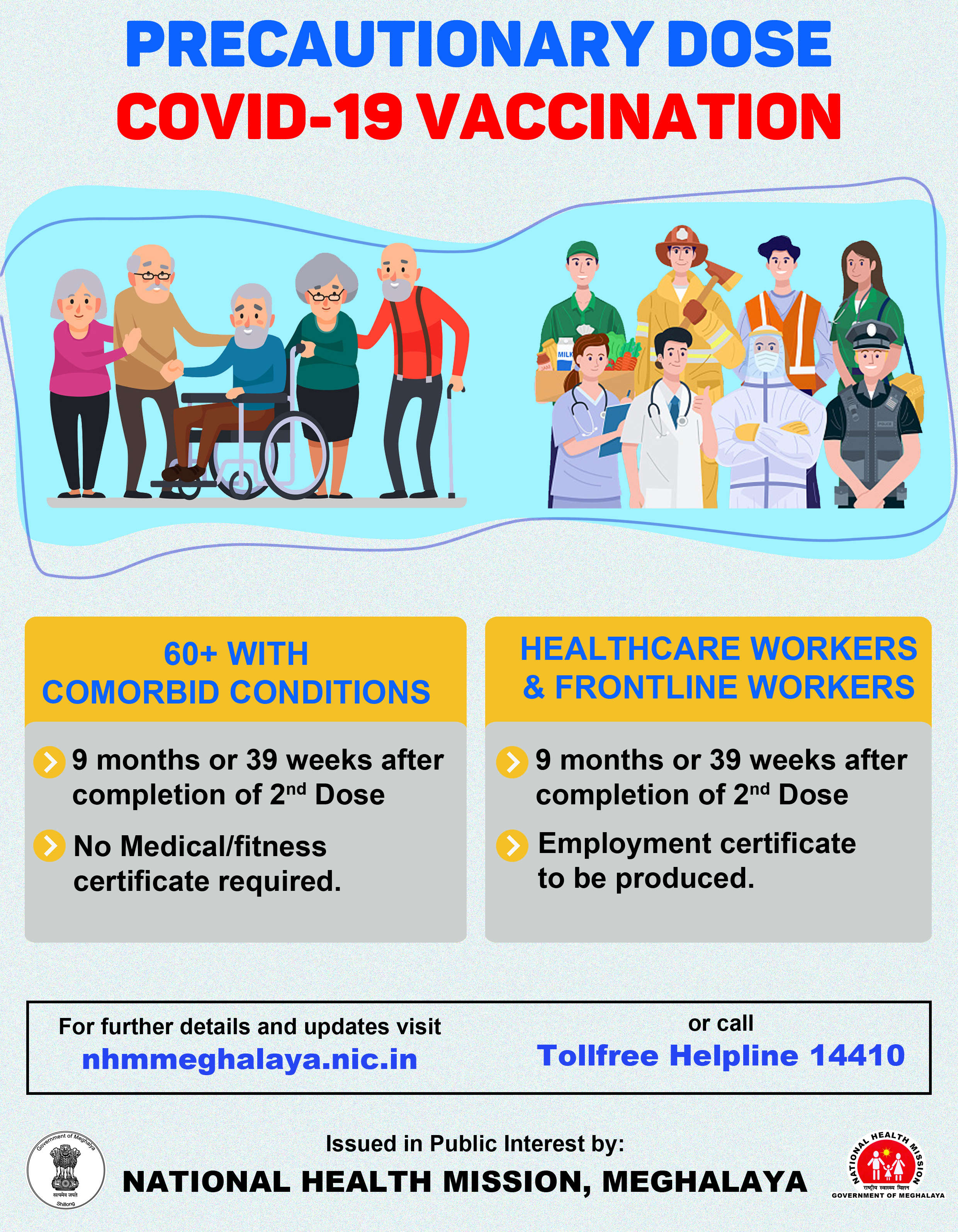
Third phase human trial of COVID-19 vaccine to start soon
Bhubaneswar (PTI)
The third phase of the human trial of the indigenous vaccine against COVID-19, COVAXIN, will commence at a private hospital here soon, an official said.
The search for a suitable vaccine for COVID-19 has almost come to the final stage, Dr E Venkata Rao, Principal Investigator in the COVAXIN human trial and Professor in the Department of Community Medicine at the Institute of Medical Sciences and SUM Hospital said on Sunday.
Indian Medical Service (IMS) and SUM Hospital are among the 21 medical institutes selected across the country by the Indian Council for Medical Research (ICMR) for the third phase trial.
The indigenous vaccine, being developed by ICMR and Bharat Biotech, has received the approval of the Central Drugs Standard Control Organisation (CDSCO) for initiating the third phase trial.
Dr Rao said we would recruit healthy volunteers for the trial, including health workers.
The volunteers would be followed up over a considerable time to look at the efficacy of the vaccine in preventing the development of the Corona disease, he said
The interested volunteers for the project could enrol themselves for the trial by registering online at www.ptctu.soa.ac under the section register for clinical trials, he said.